全部商品分类
具有α-H的醛或酮,在碱催化下生成碳负离子,然后碳负离子作为亲核试剂对醛或酮进行亲核加成,生成β-羟基醛,β-羟基醛受热脱水生成α-β不饱和醛或酮。在稀碱或稀酸的作用下,两分子的醛或酮可以互相作用,其中一个醛(或酮)分子中的α-氢加到另一个醛(或酮)分子的羰基氧原子上,其余部分加到羰基碳原子上,生成一分子β-羟基醛或一分子β-羟基酮。这个反应叫做羟醛缩合或醇醛缩合。通过醇醛缩合,可以在分子中形成新的碳碳键,并增长碳链。
醛与醛之间的加成反应是羟醛缩合反应:一个醛的α位碳加成到另一个羰基的碳上,如乙醛:
CH3CHO+CH3CHO==CH3CH(OH)CH2CHO
没有α氢的醛不能发生羟醛缩合反应,如甲醛。在碱性条件下,没有α氢的醛之间发生自氧化还原反应(康尼扎罗反应),生成一分子醇和一分子酸:
HCHO+HCHO==CH3OH+HCOOH...

'Aldol' is an abbreviation of aldehyde and alcohol. When the enolate of an aldehyde or a ketone reacts at the α-carbon with the carbonyl of another molecule under basic or acidic conditions to obtain β-hydroxy aldehyde or ketone, this reaction is called Aldol Reaction.

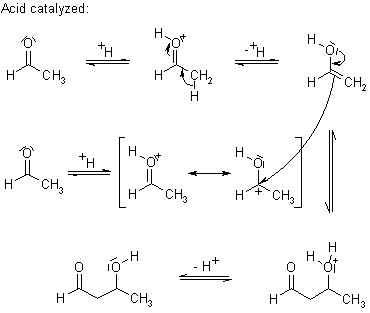
Under conditions of kinetic control, the mixed Aldol Addition can be used to prepare adducts that are otherwise difficult to obtain selectively. This process begins with the irreversible generation of the kinetic enolate, e.g. by employing a sterically hindered lithium amide base such as LDA (lithium diisopropylamide). With an unsymmetrically substituted ketone, such a non-nucleophilic, sterically-demanding, strong base will abstract a proton from the least hindered side. Proton transfer is avoided with lithium enolates at low temperatures in ethereal solvents, so that addition of a second carbonyl partner (ketone or aldehyde) will produce the desired aldol product.
